

Section 1.1.What is radiation?

Natural radiation levels may vary from place to place.
Section 1.1.What is radiation?
Radiation is energy that moves from one place to another in the form of waves or particles. Most radiation:
 Cannot be heard
Cannot be heard
 Cannot be seen
Cannot be seen
 Cannot be smelled
Cannot be smelled
 Cannot be tasted
Cannot be tasted
 Cannot be felt
Cannot be felt
However, we can use instruments to detect or measure it.
Section 1.2.Atomic tidbits
Atom
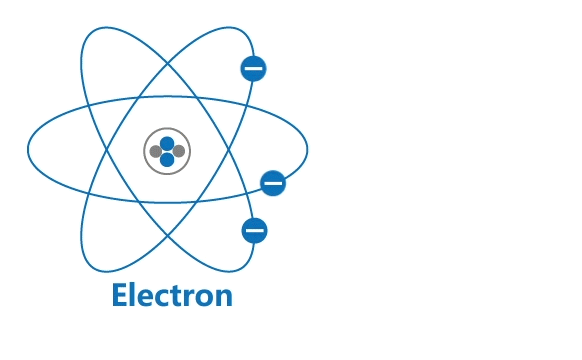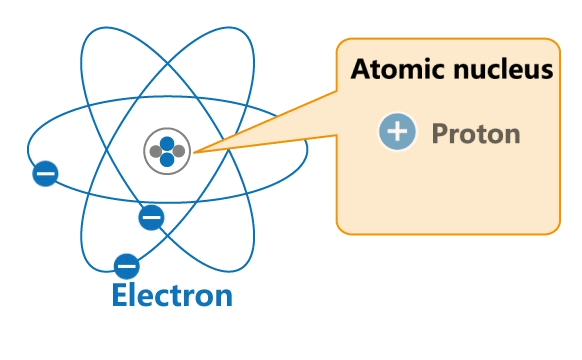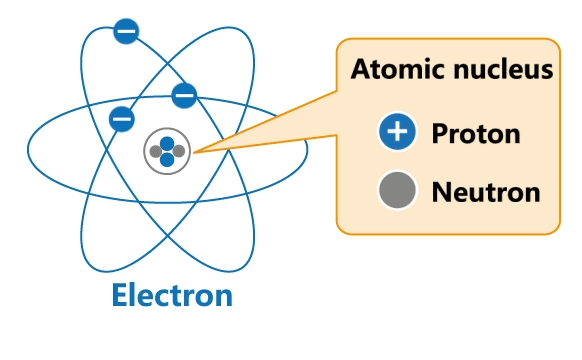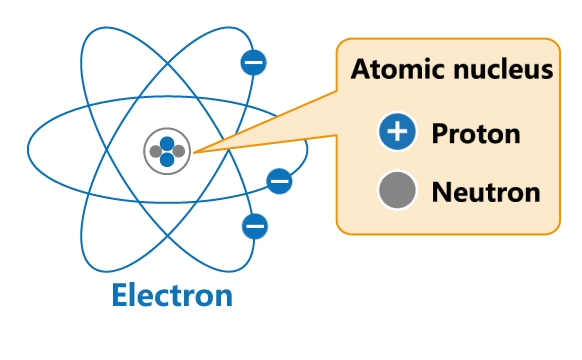

All materials are made up of tiny particles called atoms. Each atom has a nucleus and a surrounding cloud of electrons. Uncharged neutrons and positively charged protons are confined inside a nucleus, while negatively charged electrons revolve around the nucleus in orbits.
Decay


Most atomic nuclei are stable and will maintain their original states for a prolonged period. However, some nuclei, in particular those bigger in size, are indeed unstable. An unstable (radioactive) nucleus can become more stable after emitting particles and energy. This process is called decay.
Section 1.2.Atomic tidbits
Half-life
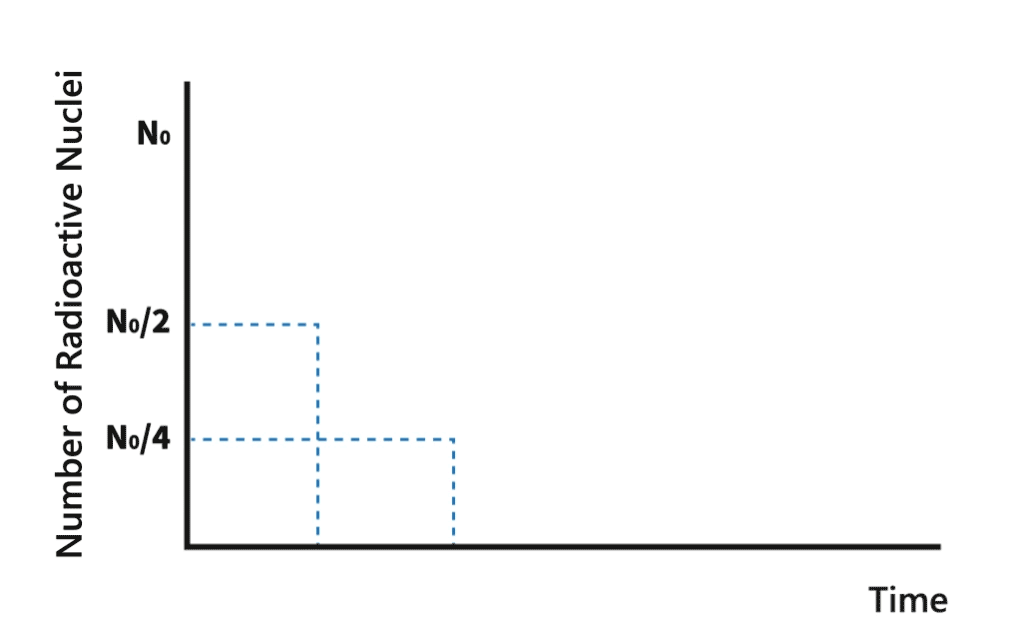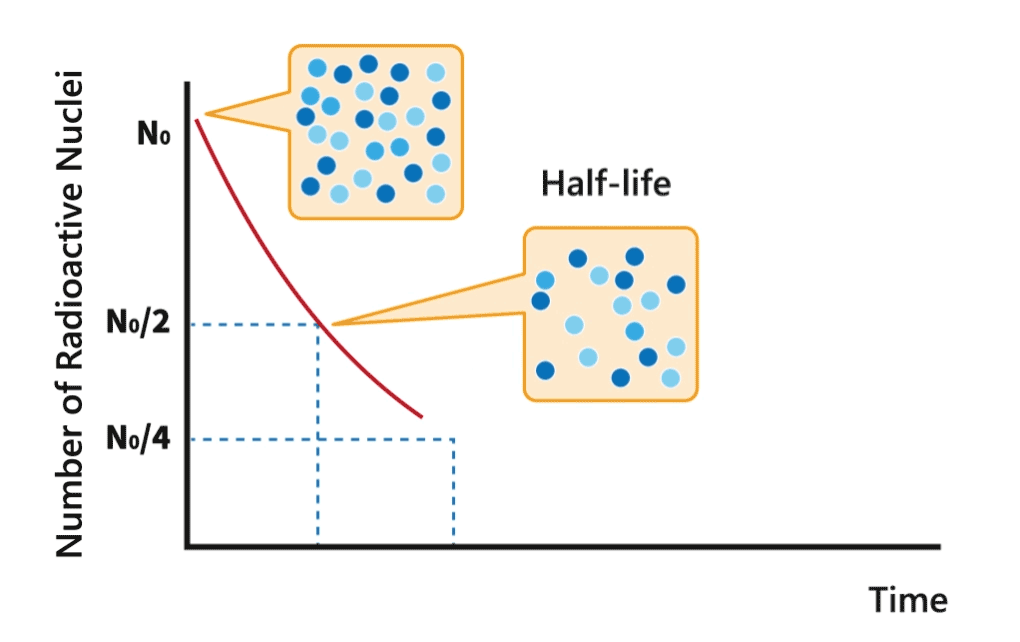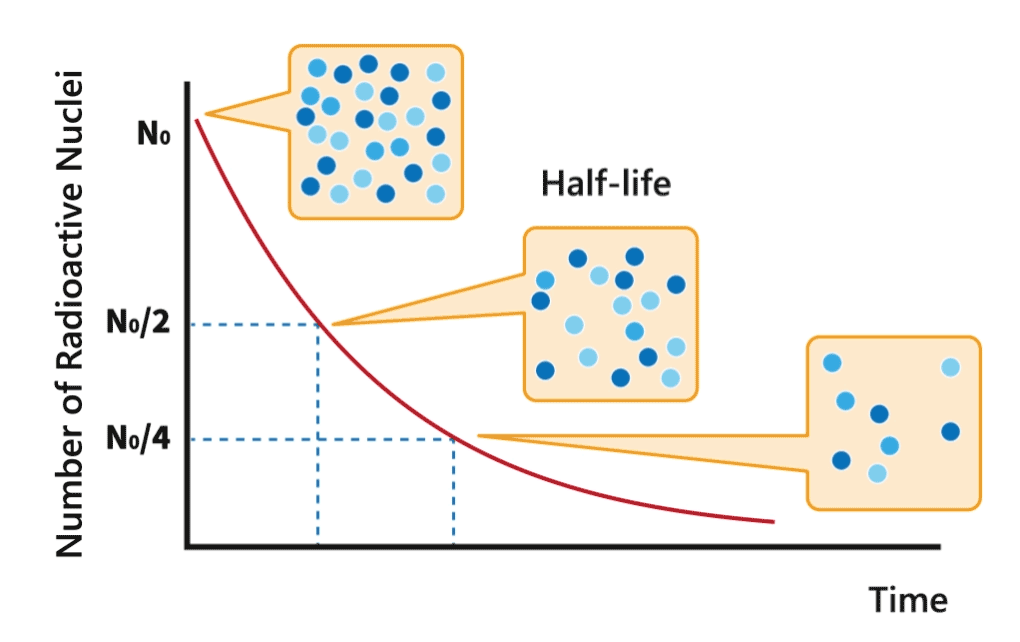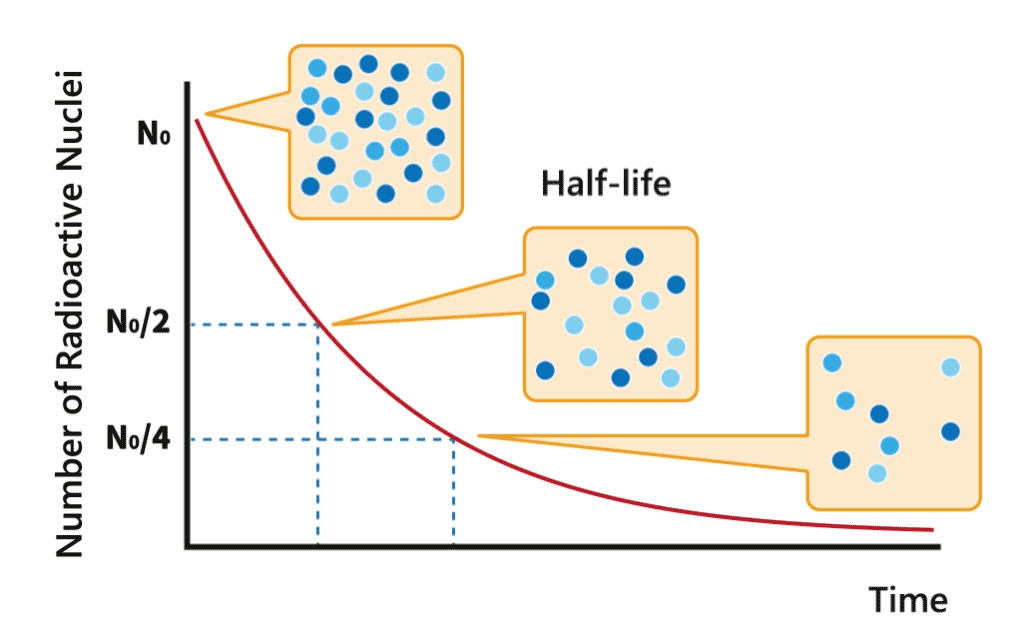

During a decay process, the number of nuclei of radionuclides gradually decreases. The time required for the number to decrease to half of its original amount is called half-life of the nuclide.

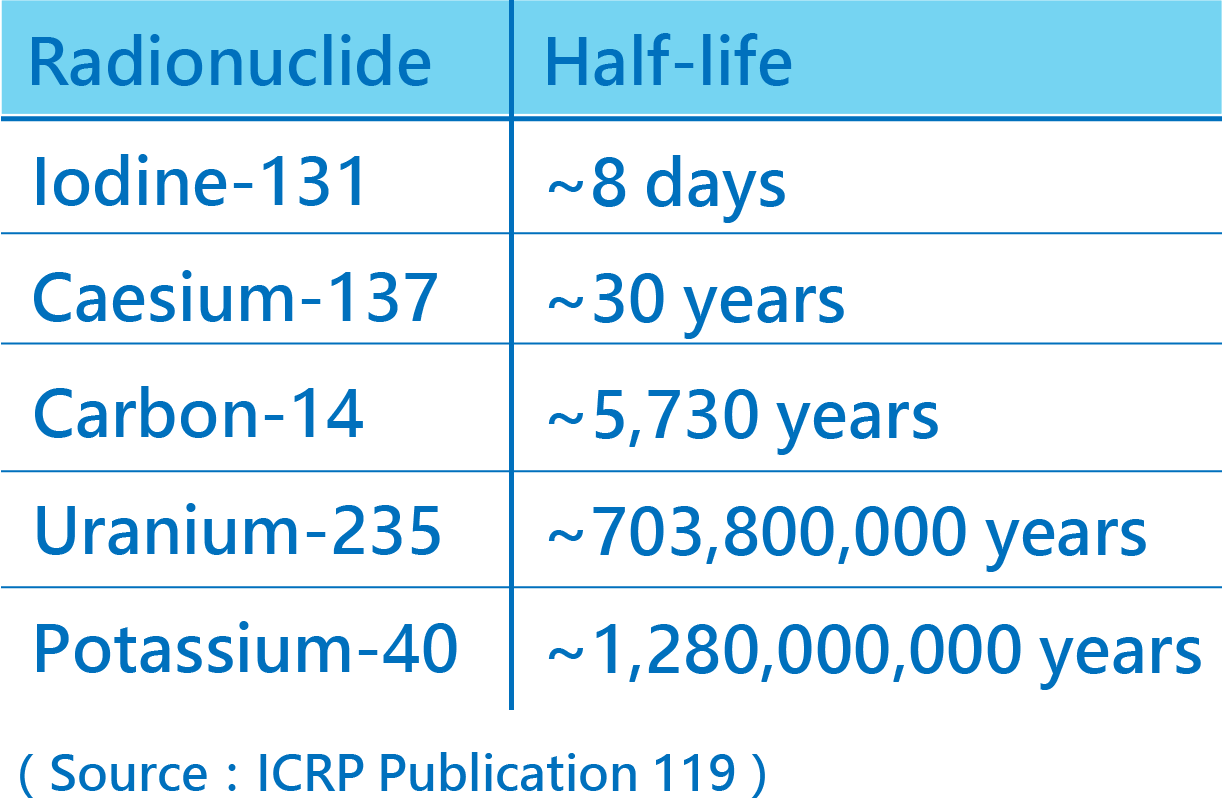
Each radionuclide has a characteristic half-life. The half-lives of radionuclides can vary from millionths of a second to over million years.
Section 1.2.Atomic tidbits
Nuclear Energy
Nuclear energy is the energy released when the structure of an atomic nucleus changes, either under nuclear fission or nuclear fusion.
Nuclear fission
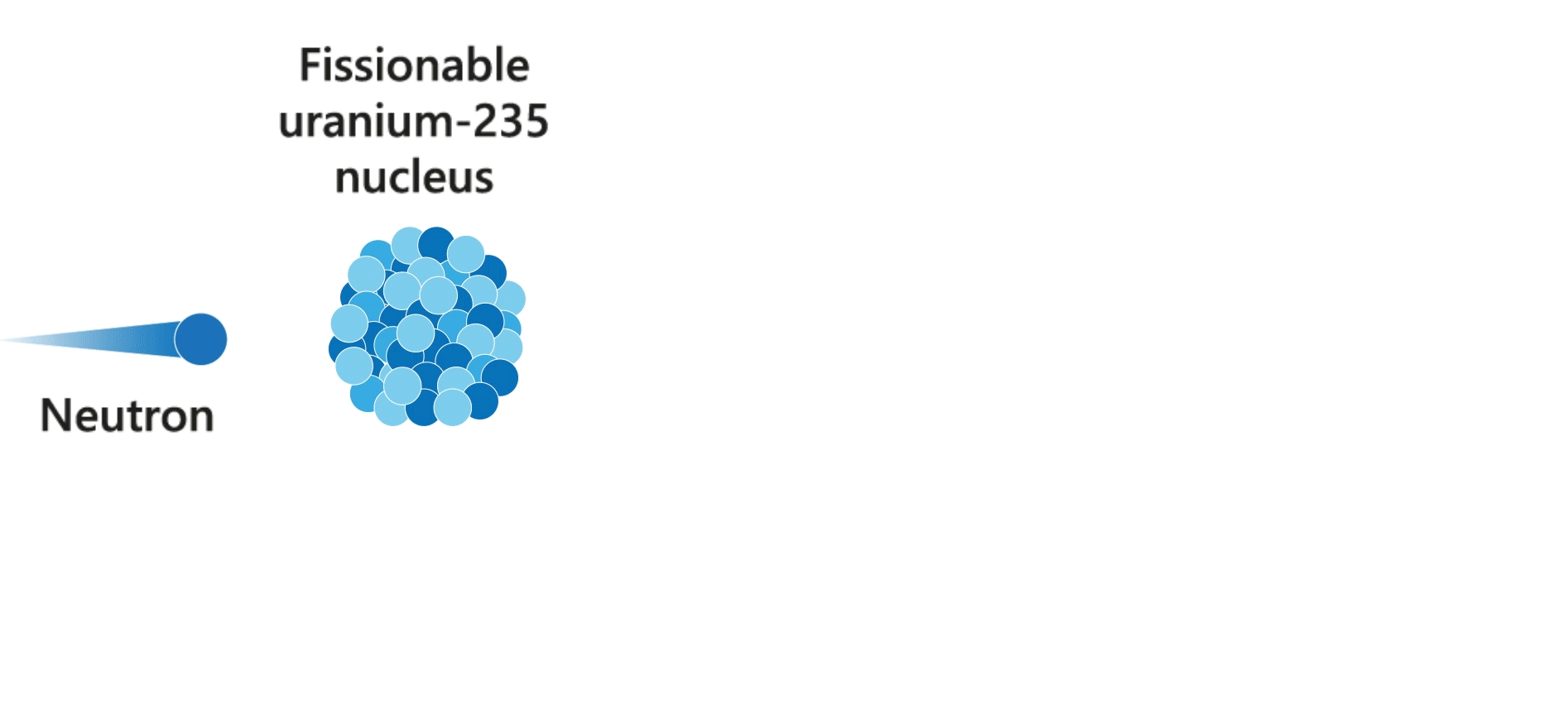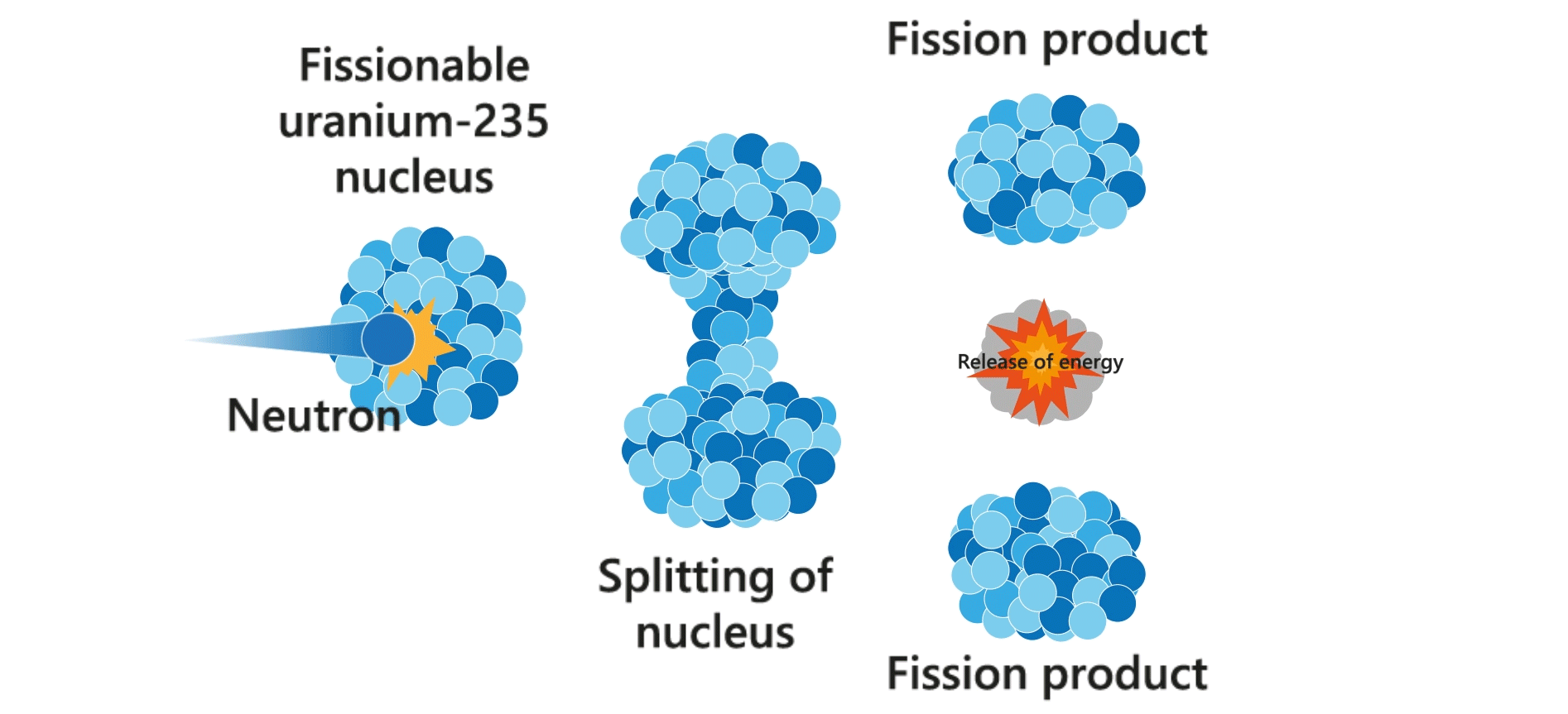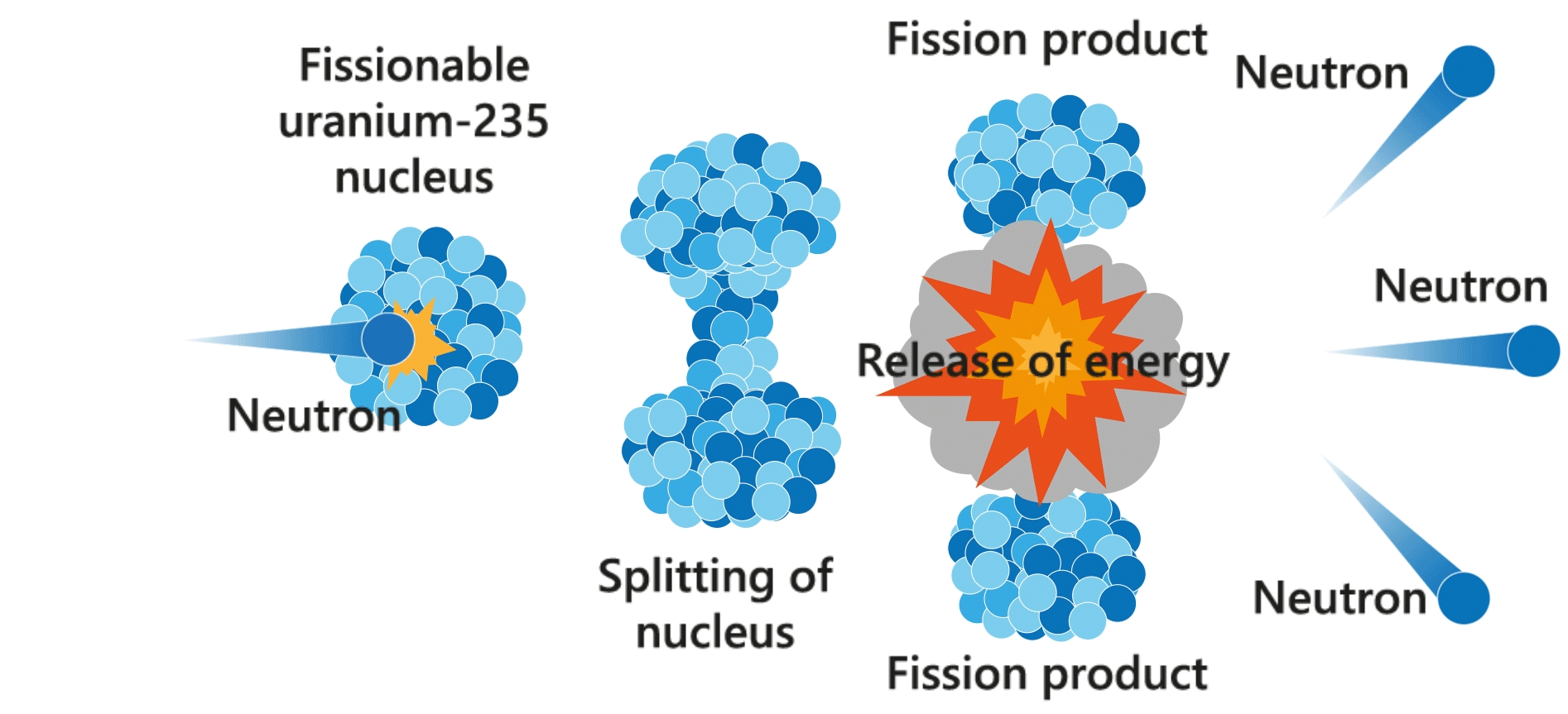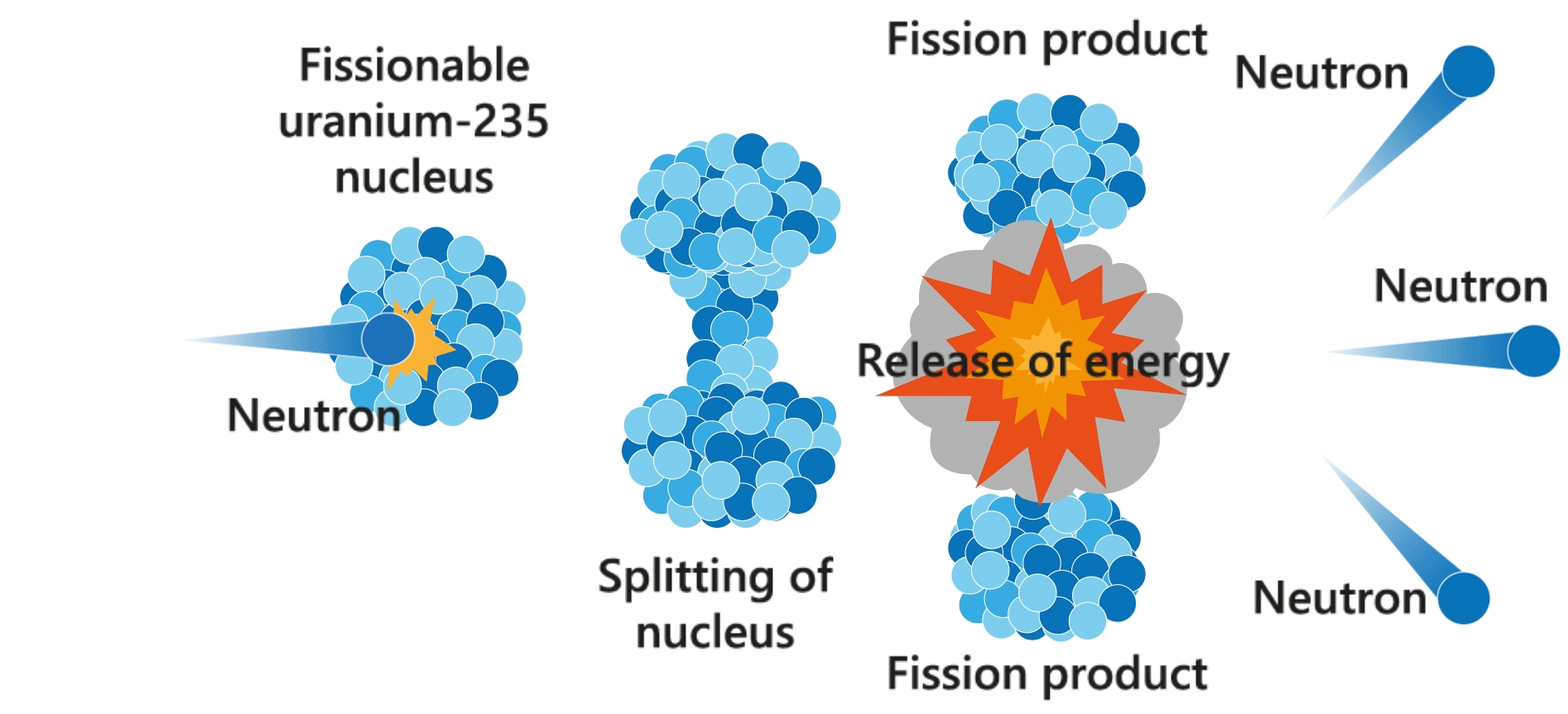
Nuclear fission is a process in which the nucleus of an atom (e.g. uranium-235) splits into lighter nuclei (daughter nuclei), accompanied with the release of energy. The process is usually triggered by a neutron.

Nuclear fusion
Nuclear fusion is the fusion of two or more atomic nuclei to form a heavier nucleus and other particles. Like nuclear fission, nuclear fusion produces energy. Deuterium and tritium, both heavy forms of hydrogen, are common materials for nuclear fusion.


Section 1.2.Atomic tidbits
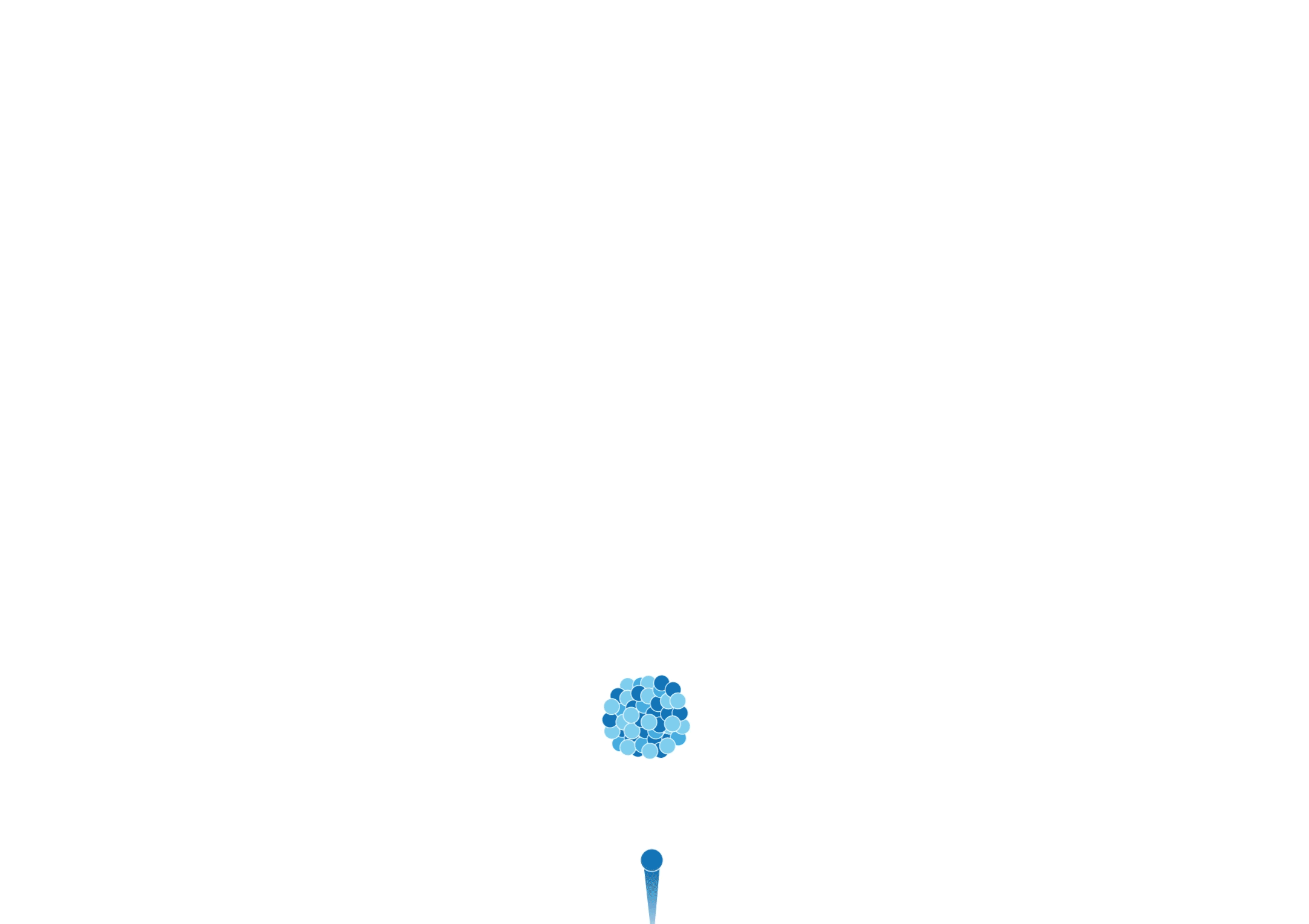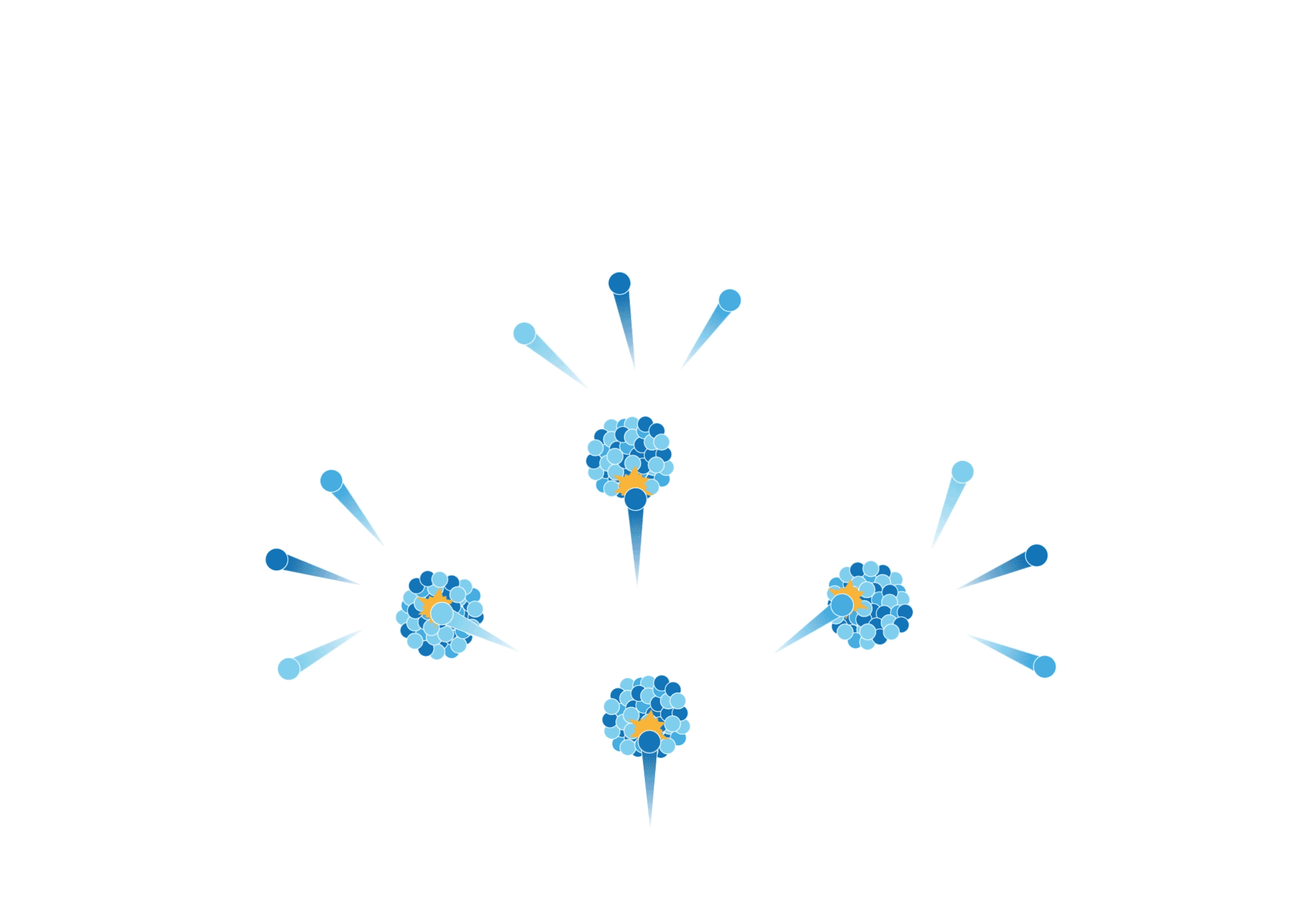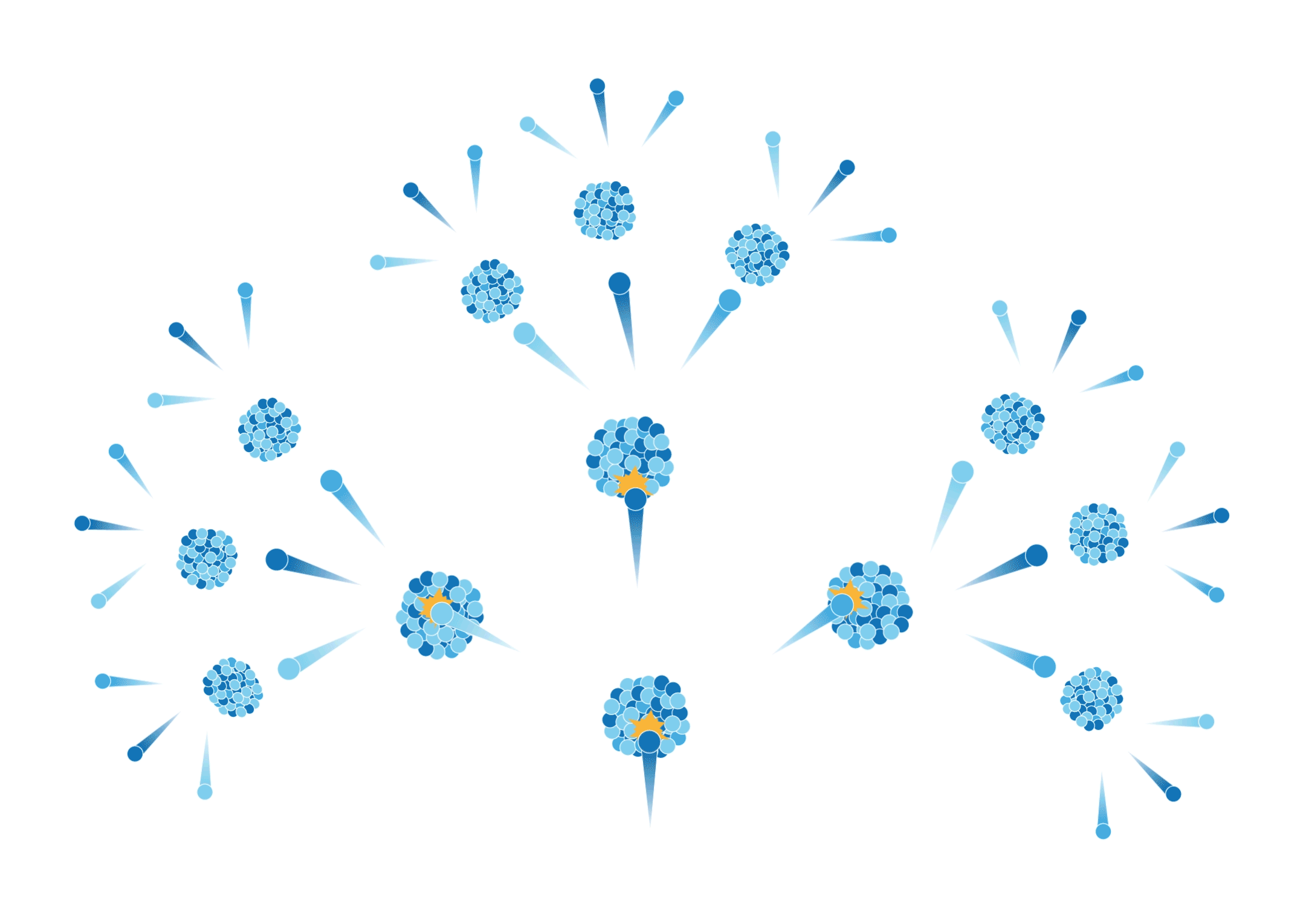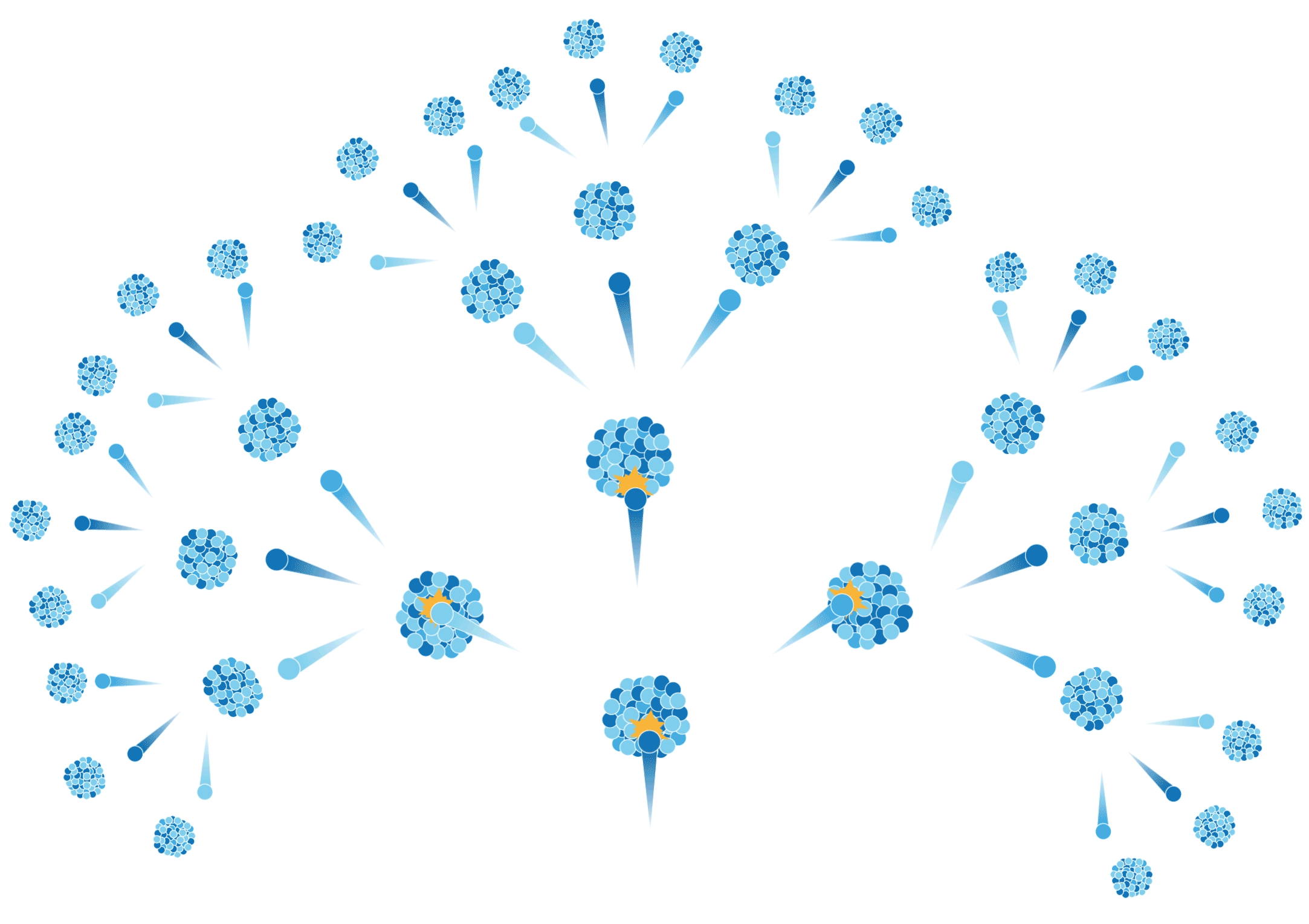
Chain reaction
The neutrons produced by the fission reactions may strike other uranium nuclei and produce more neutrons. This multiplication process (i.e. chain reaction) happens in a split second and results in the release of a large amount of heat. Nuclear power plants utilise the heat generated from controllable chain reactions to produce electricity.
Section 1.3.Ionising and non-ionising radiation
Radiation can be classified as non-ionising and ionising.
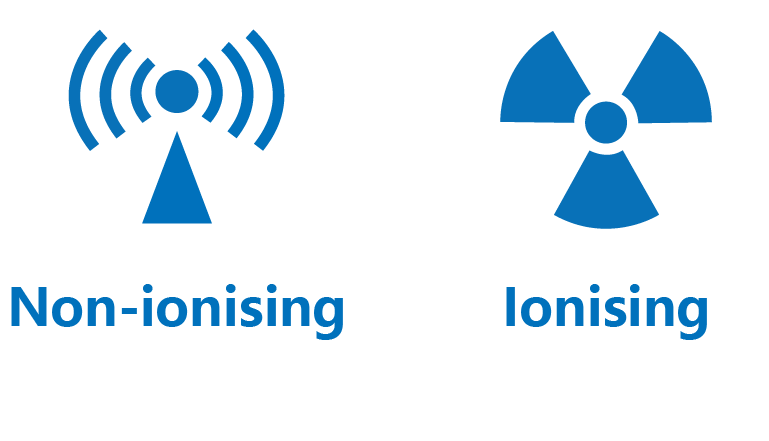

Non-ionising radiation


Non-ionising radiation contains low energy electromagnetic waves. It does not have sufficient energy to remove electrons from atoms.
Section 1.3.Ionising and non-ionising radiation
Common examples of non-ionising radiation are:
 Ultraviolet
Ultraviolet
 Visible light
Visible light
 Infrared
Infrared
 Microwave
Microwave
 Radiowave
Radiowave
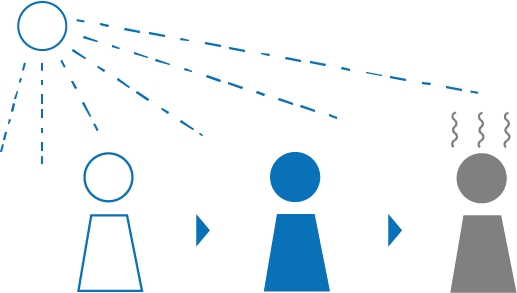
Although the energy of non-ionising radiation is lower, too much of it can still affect our health. For instance, prolonged exposure to UV radiation can cause sunburn.
Section 1.3.Ionising and non-ionising radiation
Ionising radiation
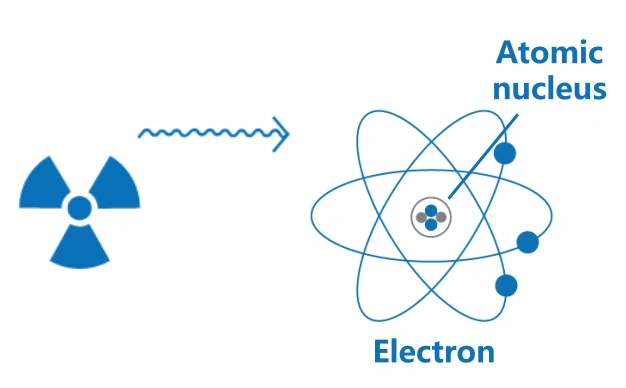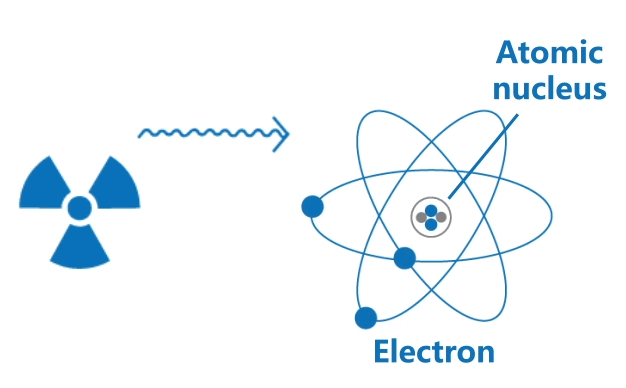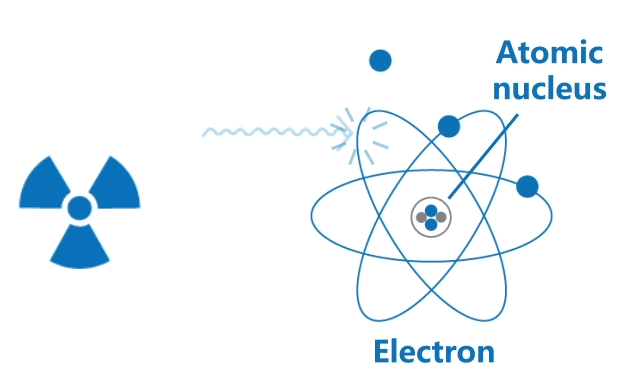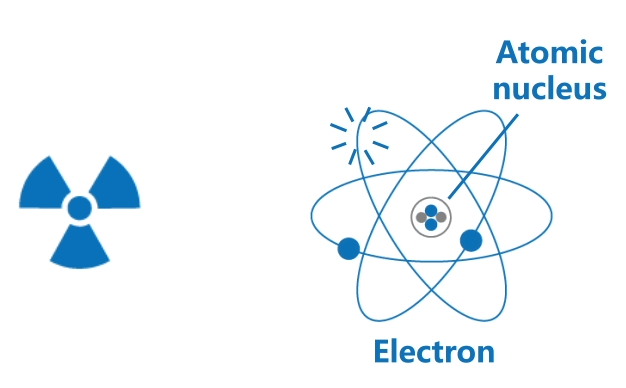

Ionising radiation includes high speed particles and high energy electromagnetic waves. Their high energy is able to knock out orbital electrons from atoms, thus generating positively charged ions and negatively charged electrons.

This ionisation process may often result in chemical changes in biological tissues, causing harm to living organisms.
The radiation discussed in the following chapters of this e-book refers to ionising radiation.
The radiation discussed in the following chapters of this e-book refers to ionising radiation.
Section 1.4.Units of radiation
The amount of radiation exposures is quantified by radiation dose. The corresponding units are Sievert (Sv) and Gray (Gy). Radioactivity is the number of decays per second of the radioactive substance or isotope. The units are Becquerel (Bq) and Curie (Ci).
Know More:
Section 1.5.Radiation dose received
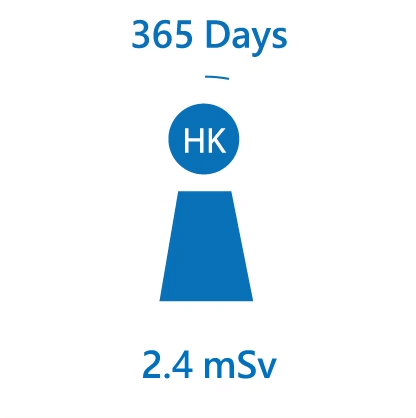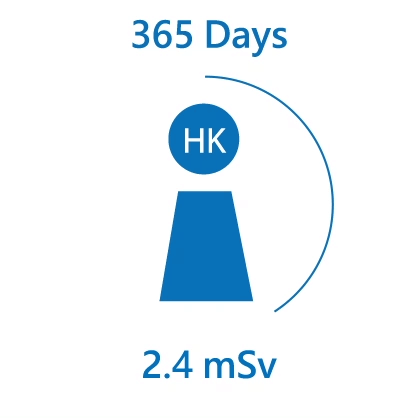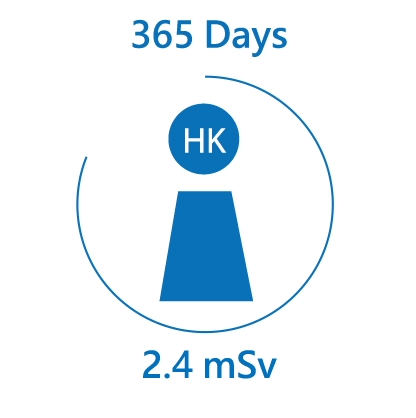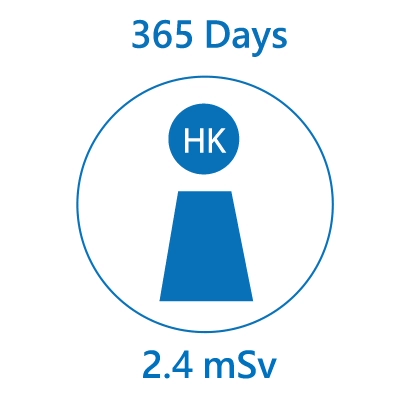
We are constantly exposed to different kinds of radiation, especially natural radiation. The average radiation dose received from natural background by an individual in Hong Kong is about 2.4 mSv per year. Over the world, the radiation dose received by an individual ranges from 2 mSv to over 10 mSv per year.
Radiation may cause damages to body cells and tissues. However, health effects are insignificant unless the absorbed dose is high. In the long-term, radiation can increase the risk of cancer. For every additional mSv of radiation exposure, the risk may increase by about 1 in 20,000.
Know More:
Section 1.6.Natural radiation
Natural radiation sources include cosmic rays, radon, radioactive materials existing in the rocks and soil of the Earth's crust, as well as radioactive materials in food and drinks.
Cosmic radiation
Cosmic radiation arises from cosmic rays. Cosmic rays mainly come from the streams of high-energy charged particles that originate from interstellar space and the Sun.
Cosmic radiation
Cosmic radiation arises from cosmic rays. Cosmic rays mainly come from the streams of high-energy charged particles that originate from interstellar space and the Sun.
 Interstellar space
Interstellar space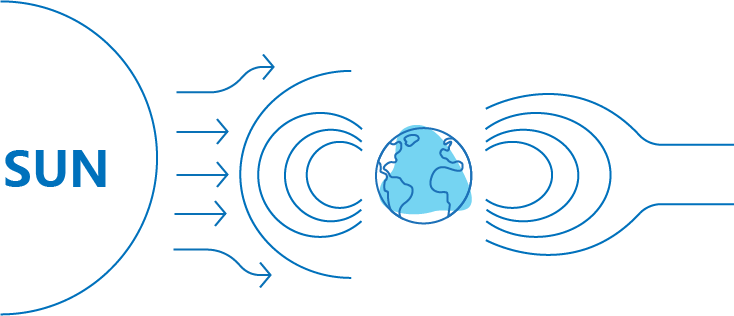 High-energy charged particles that originate from the Sun
High-energy charged particles that originate from the Sun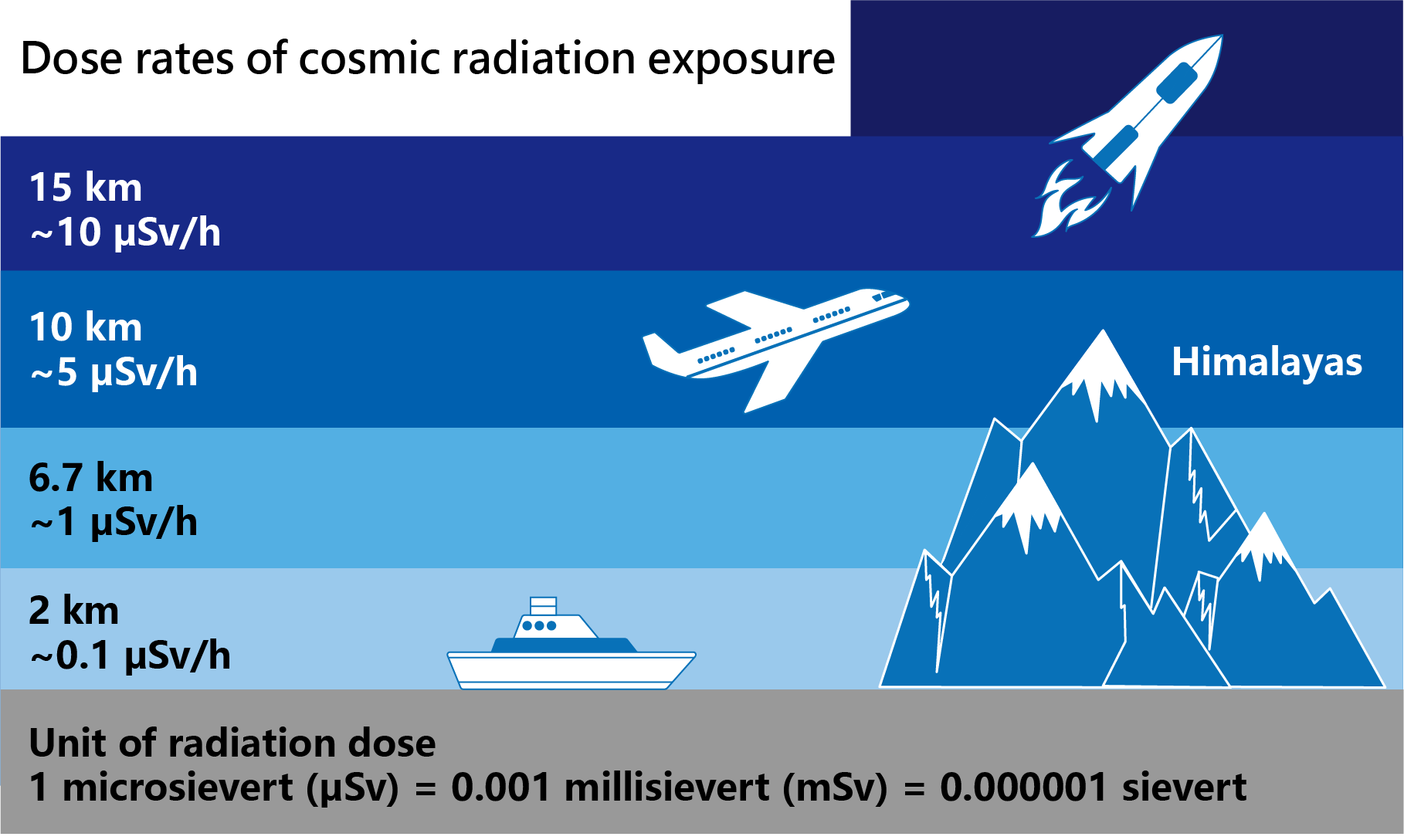
The cosmic radiation exposure is generally higher in polar regions and lower near the equator. Locations in higher altitude will also have higher level of cosmic radiation.
Section 1.6.Natural radiation
Rocks and soil

The Earth itself is a source of terrestrial radiation. Radioactive materials such as uranium, thorium, potassium and the radionuclides from their decay products exist naturally in the rocks and soil of the Earth's crust. The terrestrial radiation varies from place to place. Locations with higher concentrations of uranium and thorium generally have higher terrestrial radiation.

The Earth itself is a source of terrestrial radiation. Radioactive materials such as uranium, thorium, potassium and the radionuclides from their decay products exist naturally in the rocks and soil of the Earth's crust. The terrestrial radiation varies from place to place. Locations with higher concentrations of uranium and thorium generally have higher terrestrial radiation.
Radon
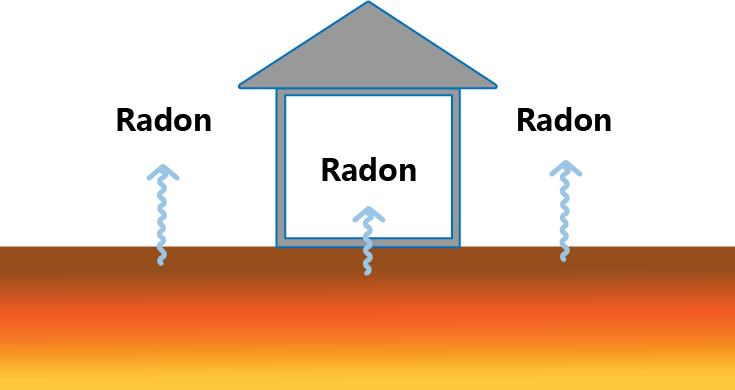
Radon is a colorless, tasteless, odorless radioactive gas mainly produced by the decay of natural radionuclide uranium-238 in soils, rocks and walls. Radon generated from the ground releases into the air, and can even enter indoor.

Radon is a colorless, tasteless, odorless radioactive gas mainly produced by the decay of natural radionuclide uranium-238 in soils, rocks and walls. Radon generated from the ground releases into the air, and can even enter indoor.
Section 1.6.Natural radiation
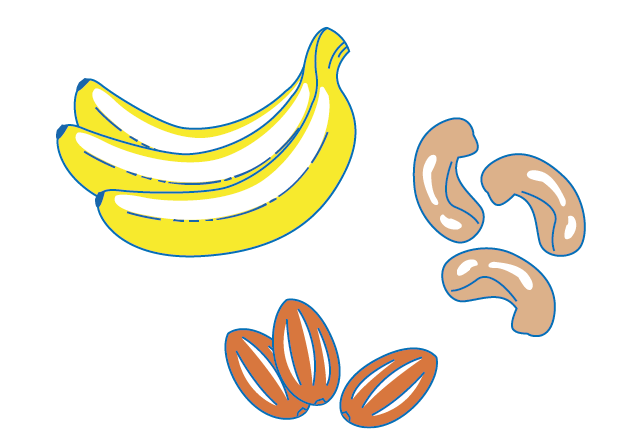
Food and drinks
Food and drinks are also naturally radioactive. Radioactive materials in soil and water can be transferred to plants and then to animals. For example, potassium-40 can be found in bananas and nuts.

Section 1.7.Artificial radiation
Artificial radiation sources include:
- medical use of radiation;
- nuclear power plants;
- nuclear industrial incidents;
- nuclear weapon tests; and
- products which produce radiation.
The following are some products that contain trace amounts of radiation:
Cigarette

Ceramic ware containing radioactive uranium dye
 Old style ionisation smoke detectors
Old style ionisation smoke detectors

Cigarette

Ceramic ware containing radioactive uranium dye
 Old style ionisation smoke detectors
Old style ionisation smoke detectorsSection 1.8.Worldwide distribution of radiation exposure
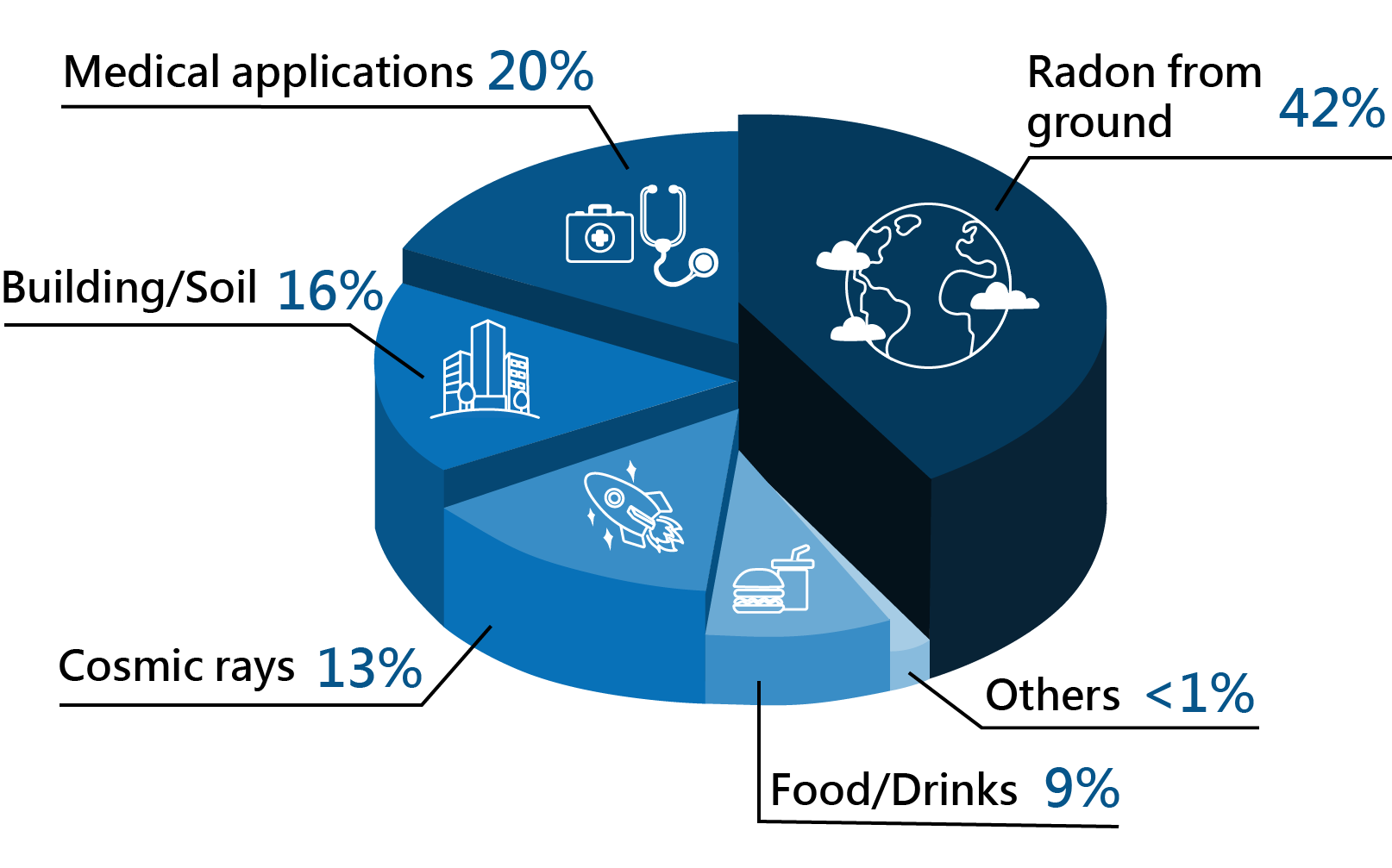
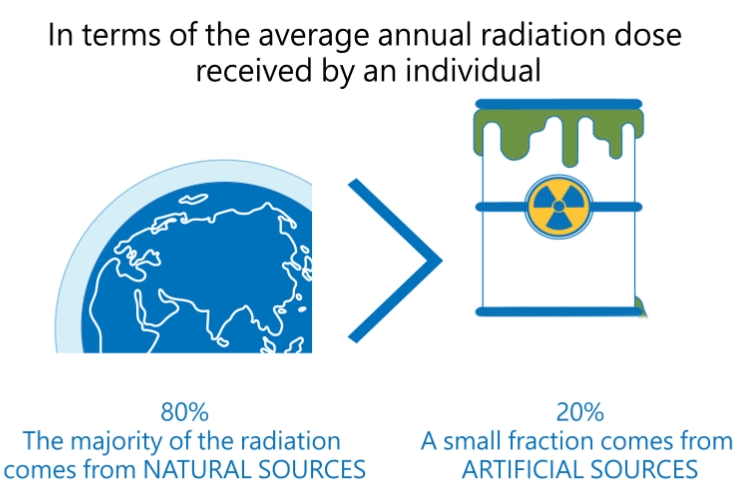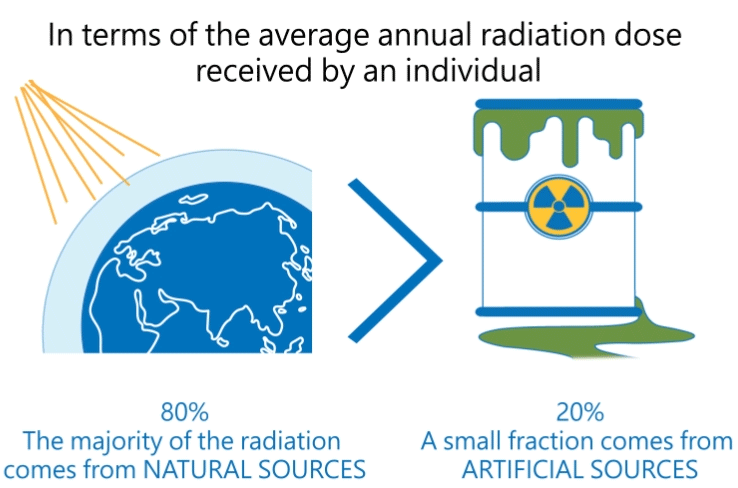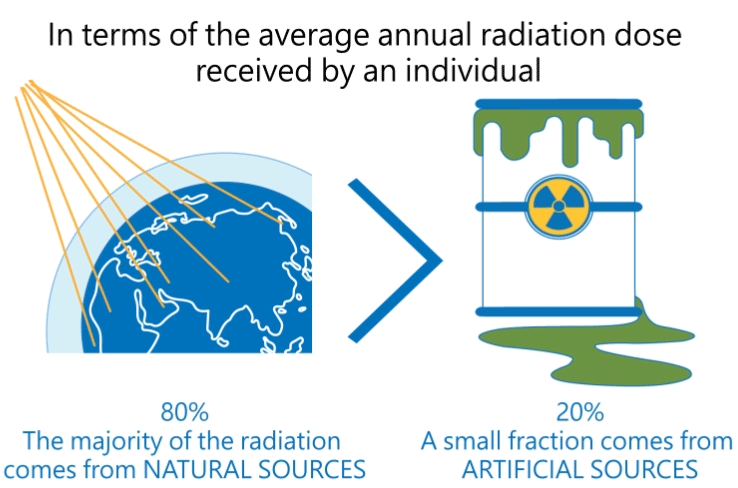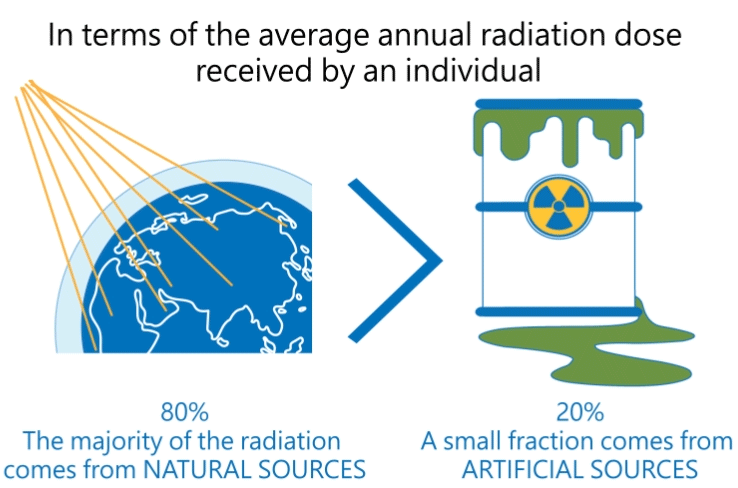
Radiation produced by human activities typically accounts for about 20 percent of public radiation exposure, mainly from medical applications (Reference: Radiation Effects and Sources, UNEP, 2016).
The other 80 percent comes from natural sources, mainly from terrestrial origin.
The other 80 percent comes from natural sources, mainly from terrestrial origin.



